Issue Date: September 3, 2012
A Nonprofit Network For Tuberculosis Drugs
News Channels: Biological SCENE
Keywords: tuberculosis, nonprofits, neglected diseases, pharmaceuticals, antibiotics
If it takes a village to raise a child, it can take a worldwide network of villages to rear a new drug. As more nonprofits shoulder the responsibility of developing better, safer, and cheaper drugs for neglected diseases such as tuberculosis and malaria, they are operating like virtual biotech firms. The Global Alliance for TB Drug Development (TB Alliance), dedicated to finding new treatments for tuberculosis, is a prime example of how knowledgeable scientists can run a global network to refill a once-empty pipeline.
The TB Alliance’s panoply of partners contributes along every step of a drug’s life cycle: basic science, high-throughput screening, medicinal chemistry, preclinical studies, clinical testing, process development, and large-scale manufacturing. The alliance teams with academics, other nonprofits, big pharma, biotechs, and for-profit contract research and contract manufacturing organizations. “Everything about the TB Alliance is collaboration, whether you’re working with a CRO, a CMO, or a biotech institute,” says Christopher B. Cooper, the alliance’s senior director of chemistry.
To manage and prioritize that work, done under the strictest of budgets, the TB Alliance relies on its staff’s experience in industry. Most of the alliance’s staffers are “dyed-in-the-wool big pharma,” Cooper says. “We have experience, contacts, and knowledge about the molecule to drive research.”
Cooper joined the TB Alliance in 2009, after more than 20 years of industrial drug discovery, first at Pfizer and later at Bristol-Myers Squibb. Working for a nonprofit has been a big transition, he acknowledges. “In a big pharma setting, you’ll have an entire division of process chemists, a whole range of expertise that you can draw from,” Cooper says. The TB Alliance, in contrast, is entirely virtual. “The minus is I don’t have 20–30 process chemists down the hall here, but the plus is we can then tap into global expertise.”
The TB Alliance’s networked approach seems to be paying off. Ten years ago, the cupboard of TB drug candidates was practically bare; today, nearly two dozen compounds are in preclinical or clinical testing, many due directly to the alliance’s efforts.
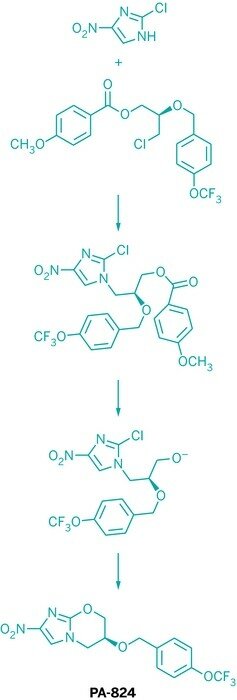
The development of PA-824, currently in Phase II studies as part of a three-drug TB regimen, illustrates how the TB Alliance puts its mosaic of partners to work.
PA-824 was discovered in the late 1990s at Seattle-based PathoGenesis. The initial bench-level synthesis of the compound, a nitroimidazole, was quick and dirty; in fact, the route involved an intermediate that was quite dangerous. “You are taking something explosive and making it more explosive,” Cooper notes.
PathoGenesis was later acquired by Chiron, which in turn was purchased by Novartis. When Chiron licensed PA-824 to the TB Alliance in 2002, it was clear process chemistry would be required to find a safer production route for clinical trials. The nonprofit enlisted the contract chemistry firm Cambridge Major Laboratories for the early work. When it came time to develop material for Phase I studies, initiated in 2005, the nonprofit turned to India’s Dr. Reddy’s Laboratories.
Now, to make the drug as cheaply and safely as possible, the TB Alliance has expanded its network to include several other companies. It hired the contract research firm Shanghai ChemPartner to further refine the process chemistry and ChemPartner’s manufacturing arm, China Gateway Pharmaceutical Development, for scale-up work. WuXi PharmaTech, Dr. Reddy’s, and GVK Bio are among the firms under consideration for large-scale manufacturing.
As it shepherds PA-824 through the pipeline, the alliance has benefited from the expertise of its scientific advisory committee. Adviser Paul J. Reider, a Princeton University professor who previously led process chemistry at Merck & Co. and small-molecule drug discovery at Amgen, has helped vet processes proposed by partners, Cooper says. Separately, the nonprofit funded a postdoctoral associate who worked in the labs of Reider and fellow Princeton chemistry professor Erik J. Sorensen to devise an elegant four-step synthesis for the nitroimidazole (J. Org. Chem., DOI: 10.1021/jo1015807).
The nonprofit hopes to settle on its manufacturing route within the next six months. That process will be shared with all of the partners for the production of PA-824 for Phase III studies and beyond.
The elbow grease that went into finding a good route to PA-824 should pay dividends as the nonprofit pushes forward TBA-354, a next-generation compound that itself was made possible by strong collaborations.
When the TB Alliance set out to find a follow-up compound to PA-824, it turned to a team of medicinal chemists at New Zealand’s Auckland Cancer Society Research Centre that had experience with nitroheteroaromatics. Led by William A. Denny, the team generated close to 1,000 analogs of PA-824. Researchers at the University of Illinois, Chicago, then studied the most active compounds in mouse models of TB to determine which of them might be more effective than PA-824.
TBA-354 emerged as the winner in October 2011, becoming the first preclinical drug candidate that the alliance generated and advanced internally, Cooper notes. The nonprofit expects to complete preclinical studies on TBA-354 by early 2013. Soon thereafter it will ask the Food & Drug Administration for permission to begin human studies.
The TB Alliance’s work on nitroimidazoles has even more legs, thanks to the collaborative nature of the neglected disease field. The Drugs For Neglected Diseases initiative (DNDi) was interested in evaluating the next-generation nitroimidazoles generated by the Auckland group in models of leishmaniasis, a disease caused by parasites that live in sand flies.
DNDi, whose North American offices share a floor with the TB Alliance in New York City, now has a preclinical drug candidate as a direct result of the alliance’s work with the Auckland team, Cooper says. Moreover, the alliance has already shared with DNDi the process chemistry improvements that made manufacturing PA-824 and TBA-354 safer and cheaper.
- Chemical & Engineering News
- ISSN 0009-2347
- Copyright © American Chemical Society