Issue Date: July 30, 2012
Turmeric-Derived Compound Curcumin May Treat Alzheimer’s
News Channels: Biological SCENE
Keywords: alternative medicine, dietary supplements, curcumin, tumeric, Alzheimer’s disease

More than 5 million people in the U.S. currently live with Alzheimer’s disease. And according to the Alzheimer’s Association, the situation is only going to get worse.
By 2050, the nonprofit estimates, up to 16 million Americans will have the memory-robbing disease. It will cost the U.S. $1.1 trillion annually to care for them unless a successful therapy is found.
Pharmaceutical companies have invested heavily in developing Alzheimer’s drugs, many of which target amyloid-β, a peptide that misfolds and clumps in the brains of patients. But so far, no amyloid-β-targeted medications have been successful. Expectation for the most advanced drugs—bapineuzumab from Pfizer and Johnson & Johnson and solanezumab from Eli Lilly & Co.—are low on the basis of lackluster data from midstage clinical trials. That sentiment was reinforced last week when bapineuzumab was reported to have failed the first of four Phase III studies.
Even if these late-stage hopefuls do somehow work, they won’t come cheap, says Gregory M. Cole, a neuroscientist at the University of California, Los Angeles. These drugs “would cost patients tens of thousands of dollars per year,” he estimates. That hefty price tag stems from bapineuzumab and solanezumab being costly-to-manufacture monoclonal antibodies against amyloid-β.
“There’s a great need for inexpensive Alzheimer’s treatments,” as well as a backup plan if pharma fails, says Larry W. Baum, a professor in the School of Pharmacy at the Chinese University of Hong Kong. As a result, he says, a great many researchers have turned their attention to less pricy alternatives, such as compounds from plants and other natural sources.
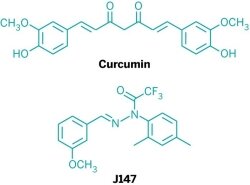
Curcumin, a spice compound derived from the rootstalk of the turmeric plant (Curcuma longa), has stood out among some of the more promising naturally derived candidates.
When administered to mice that develop Alzheimer’s symptoms, curcumin decreases inflammation and reactive oxygen species in the rodents’ brains, researchers have found. The compound also inhibits the aggregation of troublesome amyloid-β strands among the animals’ nerve cells. But the development of curcumin as an Alzheimer’s drug has been stymied, scientists say, both by its low uptake in the body and a lack of funds for effective clinical trials—obstacles researchers are now trying to overcome.
In addition to contributing to curry dishes’ yellow color and pungent flavor, curcumin has been a medicine in India for thousands of years. Doctors practicing traditional Hindu medicine admire turmeric’s active ingredient for its anti-inflammatory properties and have used it to treat patients for ailments including digestive disorders and joint pain.
Only in the 1970s did Western researchers catch up with Eastern practices and confirm curcumin’s anti-inflammatory properties in the laboratory. Scientists also eventually determined that the polyphenolic compound is an antioxidant and has chemotherapeutic activity.
Bharat B. Aggarwal, a professor at the University of Texas M. D. Anderson Cancer Center, says curcumin is an example of a pleiotropic agent: It has a number of different effects and interacts with many targets and biochemical pathways in the body. He and his group have discovered that one important molecule targeted and subsequently suppressed by curcumin is NF-κB, a transcription factor that switches on the body’s inflammatory response when activated (J. Biol. Chem., DOI: 10.1074/jbc.270.42.24995).
Aside from NF-κB, curcumin seems to interact with several other molecules in the inflammatory pathway, a biological activity that Aggarwal thinks is advantageous. “All chronic diseases are caused by dysregulation of multiple targets,” he says. “Chemists don’t yet know how to design a drug that hits multiple targets.” With curcumin, “Mother Nature has already provided a compound that does so.”
Curcumin’s pleiotropy also brought it to the attention of UCLA’s Cole during the early 1990s while he was searching for possible Alzheimer’s therapeutics. “That was before we knew about amyloid-β” and its full role in Alzheimer’s, he says. “We were working on the disease from an oxidative damage and inflammation point of view—two processes implicated in aging.”
When Cole and his wife, Sally A. Frautschy, also at UCLA, searched the literature for compounds that could tackle both of these age-related processes, curcumin jumped out at them. It also didn’t hurt that the incidence of Alzheimer’s in India, where large amounts of curcumin are consumed regularly, is lower than in other parts of the developing world (Lancet Neurol., DOI: 10.1016/s1474-4422(08)70169-8).
In 2001, Cole, Frautschy, and colleagues published the first papers that demonstrated curcumin’s potential to treat neurodegenerative disease (Neurobiol. Aging, DOI: 10.1016/s0197-4580(01)00300-1; J. Neurosci.2001, 8370). The researchers studied the effects of curcumin on rats that had amyloid-β injected into their brains, as well as mice engineered to develop amyloid brain plaques. In both cases, curcumin suppressed oxidative tissue damage and reduced amyloid-β deposits.
Those results, Cole says, “turned us into curcumin-ologists.”
Although the UCLA team observed that curcumin decreased amyloid plaques in animal models, at the time, the researchers weren’t sure of the molecular mechanism involved.
Soon after the team’s first results were published, Cole recalls, a colleague brought to his attention the structural similarity between curcumin and the dyes used to stain amyloid plaques in diseased brain tissue. When Cole and Frautschy tested the spice compound, they saw that it, too, could stick to aggregated amyloid-β. “We thought, ‘Wow, not only is curcumin an antioxidant and an anti-inflammatory, but it also might be an anti-amyloid drug,’ ” he says.
In 2004, a group in Japan demonstrated that submicromolar concentrations of curcumin in solution could inhibit aggregation of amyloid-β and break up preformed fibrils of the stuff (J. Neurosci. Res., DOI: 10.1002/jnr.20025). Shortly after that, the UCLA team demonstrated the same (J. Biol. Chem., DOI: 10.1074/jbc.m404751200).
As an Alzheimer’s drug, however, it’s unclear how important it is that the spice compound inhibits amyloid-β aggregation, Cole says. “When you have something that’s so pleiotropic,” he adds, “it’s hard to know” which of its modes of action is most effective.
Having multiple targets may be what helps curcumin have such beneficial, neuroprotective effects, says David R. Schubert, a neurobiologist at the Salk Institute for Biological Studies, in La Jolla, Calif. But its pleiotropy can also be a detriment, he contends.
The pharmaceutical world, Schubert says, focuses on designing drugs aimed at hitting single-target molecules with high affinity. “But we don’t really know what ‘the’ target for curcumin is,” he says, “and we get knocked for it on grant requests.”
Another problem with curcumin is poor bioavailability. When ingested, UCLA’s Cole says, the compound gets converted into other molecular forms, such as curcumin glucuronide or curcumin sulfate. It also gets hydrolyzed at the alkaline and neutral pHs present in many areas of the body. Not much of the curcumin gets into the bloodstream, let alone past the blood-brain barrier, in its pure, active form, he adds.
Unfortunately, neither Cole nor Baum at the Chinese University of Hong Kong realized the poor bioavailability until they had each launched a clinical trial of curcumin. So the studies showed no significant difference between Alzheimer’s patients taking the spice compound and those taking a placebo (J. Clin. Psychopharmacol., DOI: 10.1097/jcp.0b013e318160862c).
“But we did show curcumin was safe for patients,” Baum says, finding a silver lining to the blunder. “We didn’t see any adverse effects even at high doses.”
Some researchers, such as Salk’s Schubert, are tackling curcumin’s low bioavailability by modifying the compound to improve its properties. Schubert and his group have come up with a molecule, called J147, that’s a hybrid of curcumin and cyclohexyl-bisphenol A. Like Cole and coworkers, they also came upon the compound not by initially screening for the ability to interact with amyloid-β, but by screening for the ability to alleviate age-related symptoms.
The researchers hit upon J147 by exposing cultured Alzheimer’s nerve cells to a library of compounds and then measuring changes to levels of biomarkers for oxidative stress, inflammation, and nerve growth. J147 performed well in all categories. And when given to mice engineered to accumulate amyloid-β clumps in their brains, the hybrid molecule prevented memory loss and reduced formation of amyloid plaques over time (PLoS One, DOI: 10.1371/journal.pone.0027865).
Other researchers have tackled curcumin’s poor bioavailability by reformulating it. Both Baum and Cole have encapsulated curcumin in nanospheres coated with either polymers or lipids to protect the compound from modification after ingestion. Cole tells C&EN that by packaging the curcumin in this way, he and his group have gotten micromolar quantities of it into the bloodstream of humans. The researchers are now preparing for a small clinical trial to test the formulation on patients with mild cognitive impairment, who are at an increased risk of developing Alzheimer’s.
An early-intervention human study such as this one comes with its own set of challenges, Cole says. People with mild cognitive impairment “have good days and bad days,” he says. A large trial over a long period would be the best way to get any meaningful data, he adds.
Such a trial can cost up to $100 million, a budget big pharma might be able to scrape together but that is far out of reach for academics funded by grants, Cole says. “If you’re down at the level of what an individual investigator can do, you’re running a small trial,” he says, “and even if the result is positive, it might be inconclusive” because of its small size or short duration. That’s one of the reasons the curcumin work is slow-going, Cole contends.
The lack of hard clinical evidence isn’t stopping people from trying curcumin anyway. Various companies are selling the spice compound as a dietary supplement, both in its powdered form and in nanoformulations such as the ones Cole and Baum are working with. Indiana-based Verdure Sciences, for instance, licensed a curcumin nanoformulation from UCLA and sells it under the name Longvida (about $1.00 to $2.00 per capsule, depending on the distributor).
“There’s no proof that it works,” Cole says. “If you want to take it, you’re experimenting on yourself.” And he cautions that correct dosing for this more bioavailable form of curcumin hasn’t yet been established, so there could be safety concerns.
But on the basis of positive e-mails he’s received from caregivers and Alzheimer’s patients who are desperate for options and trying supplements, “I have some hope,” Cole says. “Maybe there’s something to curcumin after all.”
- Chemical & Engineering News
- ISSN 0009-2347
- Copyright © American Chemical Society
Curcumin contributes the yellow-golden color of turmeric but does not contribute flavor, which is why curcumin is widely used as a coloring for cosmetics, pop corn, drinks--even ice cream.
I think bioavailability may not have been the main cause of negative results in clinical trials. That's because results were positive in animal studies despite poor bioavailability. What differed between animal and human studies? Duration was one factor. 3-6 months of treatment in mice that live 18-24 months may have much greater effect than 6-12 months of treatment in humans that live 60-90 years. Memory did not significantly deteriorate among humans in the placebo group. Therefore, a longer study may be necessary to see whether curcumin slows deterioration.
As Cole mentioned, long trials are expensive. Who will pay for them? Compounds in the public domain, like curcumin, are unlikely to be tested by companies. That leaves governments and charities to fund trials. I hope they do.